
Title:Variable nanosheets for highly efficient oxygen evolution reaction
Corresponding author:Prof. Tie Wang,Tianjin University of Technology、Institute of Chemistry Chinese Academy of Sciences
First author:Dr. Xuezhi Qiao(Currently Associate Professor at Shandong University)
Energy system transition is critical to achieve carbon peaking and carbon neutrality goals, and hydrogen as a clean, sustainable energy source is an ideal alternative to fossil fuels. Among the various water-to-hydrogen technologies, electrolytic water-to-hydrogen plays a key role in the decarbonization efforts of national economies. Among them, the Oxygen Evolution Reaction (OER) is the more energy intensive reaction, and a highly active and stable OER is the key to electrochemical hydrolysis. As a non-homogeneous catalytic reaction, gas separation and reactant mass transfer at the reaction interface greatly affect the OER efficiency. The gas products adhere to the surface of the solid catalyst and gradually grow into bubbles, covering a large area of catalytic active sites. The restricted mass transfer rate greatly hinders the diffusion of liquid phase reactants and affects the reaction rate.
To address the above key scientific issues, Prof. Tie Wang's team at Tianjin University of Technology and Institute of Chemistry, Chinese Academy of Sciences, based on previous research (J. Am. Chem. Soc., 2020, 142, 9408-9414.), found that the atomic coordination and binding forces on the surface of nanosheets are weaker than those of bulk materials, and thus the mechanical properties of the materials are negatively correlated with thickness. In this study, we prepared micro- and nano-superstructured materials with small Young's modulus, flexible and deformable by tuning the synthesis conditions of the materials to accelerate gas separation on the electrode surface and mass transfer at the interface, thus increasing the rate of OER.
Two metal organic framework (MOF) templates NF-MOF1h and NF-MOF5h with different thickness and morphology were grown on the surface of nickel foam ( NF) by tuning the reaction time. aqueous-phase unstable MOF was transformed into more stable cobalt-nickel sulfides (NF-CoNiS1h and NF-CoiS5h). Scanning electron microscopy and transmission electron microscopy images showed that the morphology of the nanosheets remained consistent during the sulfidation process. Powder X Ray Diffraction (PXRD) and Raman spectroscopy confirmed that the crystal structures of the synthesized MOF and cobalt-nickel sulfides were consistent with those of Co-Ni MOF and NiCo2S4 (JCPDF:20-0782).
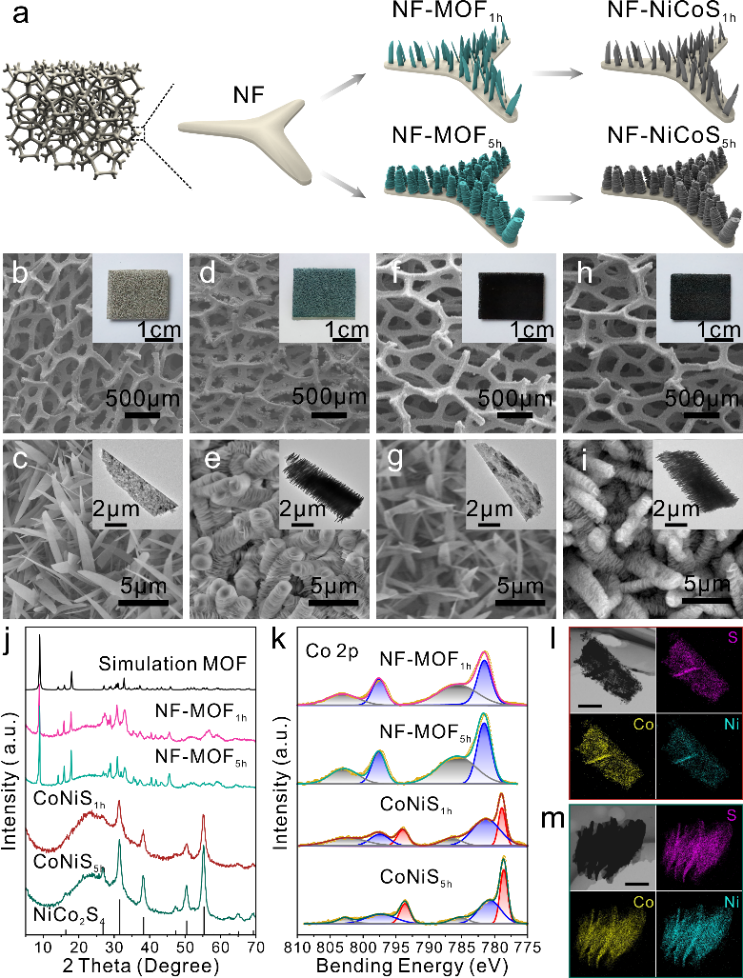
Figure 1: Material characterization
Atomic Force Microscope (AFM) height images show that the thickness of the cobalt-nickel sulfide nanosheets in NF-CoNiS1h is 34.9±2.1 nm, while the thickness in NF-CoNiS5h is 5.4±1.0 nm. The Young's modulus (E) of NF-CoNiS1h based on the Derjaguin-Mueller-Toporov ( DMT) model estimated Young's modulus (E) of NF-CoNiS1h is 3.55±0.36 GPa, while the modulus of NF-CoNiS5h is only 0.17±0.02 GPa, which is similar to that of soft and deformable materials such as DNA, rubber and polymers. The deformability of the nanosheets was verified by in situ TEM, and the thin stacked nanosheets of NF-CoNiS5h were flexible, allowing for expansion and contraction and exhibiting periodicity, while the NF-CoNiS1h nanosheets did not exhibit such expansion properties. Simulations of deformation of the materials with different thicknesses and Young's modulus using COMSOL Multiphysics show that the thinner the thickness, the smaller the modulus and the larger the offset angle and offset distance. Stacked nanosheets with a thickness of 5 nm and a Young's modulus of 0.3 GPa bend under a simulated constant electric field and show periodic variations in deflection angle and deflection displacement.
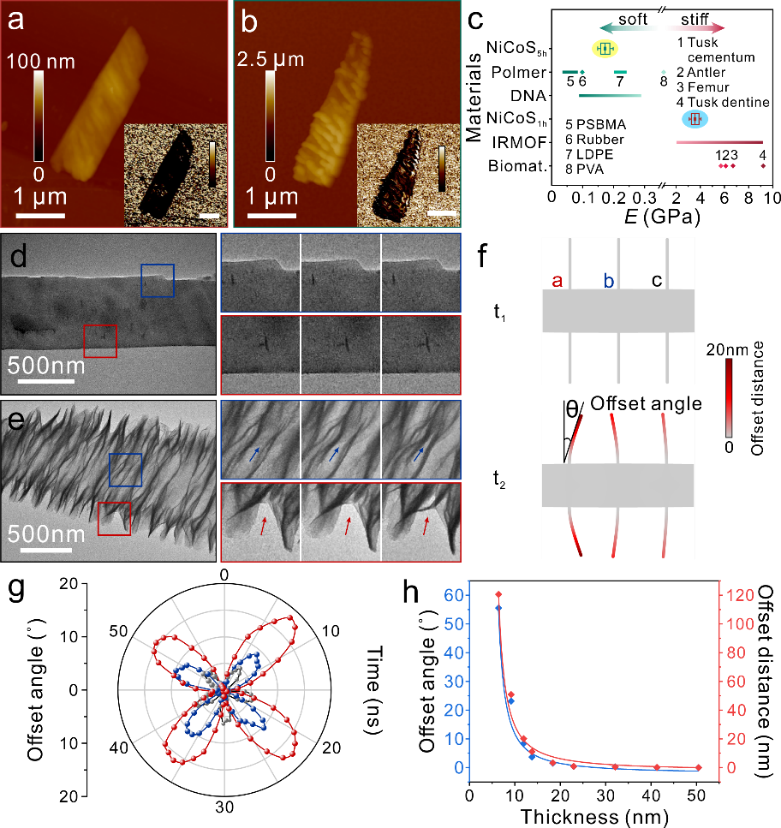
Figure 2: Mechanical properties of materials
In the chemistry of electrochemical oxygen production, the mass transfer efficiency of the electrode surface significantly affects the electrocatalytic rate. The periodic contraction and expansion of the material improves the mass transfer of reactants and products around the material in two main ways. First, the separation of bubbles is accelerated; second, the forced convection caused by the deformation reduces the concentration gradient of reactants and products. In situ monitoring of bubble detachment experiments on NF-CoNiS1h and NF-CoNiS5h during electrolysis of water showed that the bubble behavior on the NF-CoNiS5h sample was significantly different from that of NF-CoNiS1h. In NF-CoNiS1h, the gas products adhered to the surface and continued to be produced, forming larger bubbles, which only separated from the electrode when the material adhesion force was not sufficient to adhere to the bubble surface is separated. However, in NF-CoNiS5h, the bubbles were smaller and separated more quickly. The bubbles adhering to the solid electrode hinder the contact between water and catalyst, forming inactive catalytic sites and reducing the reaction rate. The integration of the concentration fields of reactants and products during the electrochemical reaction into the simulation of the material motion under the applied electric field shows that the rate of reactant consumption and product generation of the deformable nanomaterials is enhanced, and this enhancement is mainly due to the forced convection caused by deformation. Even under low Reynolds number conditions, liquids can pump reactants by such forced convection with irreversible kinetics. At the same time, the flexible nanosheets exhibit tropism, i.e., the electric field drives their active deformation to regions of higher reactant concentration to promote further catalytic reactions.
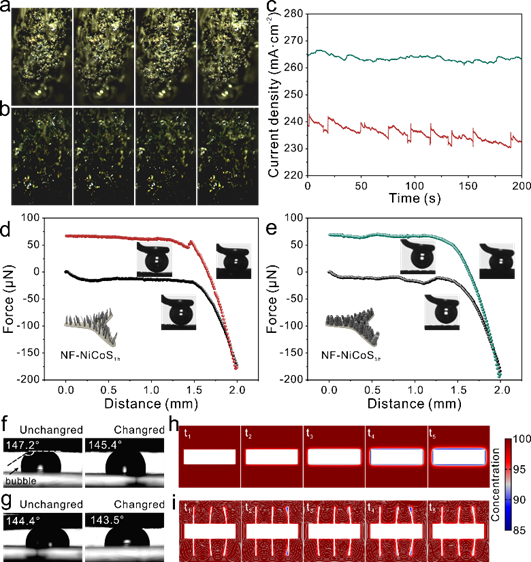
Figure 3: The bubble behavior indicated by the catalyst
Compared with MOF, the cobalt-nickel sulfide with similar morphology exhibited superior electrocatalytic activity. Notably, NF-CoNiS5h with a deformable nanosheet stacked structure shows further enhanced OER activity compared to NF-CoNiS1h, as evidenced by a significant reduction in overpotential and a significant decrease in the Tafel slope. This is attributed to the fact that the deformable stacked 3D nanosheet superstructure exposes a large number of surface active centers and accelerates the separation of bubbles at the material indication, thus promoting rapid charge transfer at the electrolyte-electrode interface. In summary, NF-CoNiS5h, a micro-nano-superstructured material with a stacked structure, is a stable and practical electrocatalyst for OER.
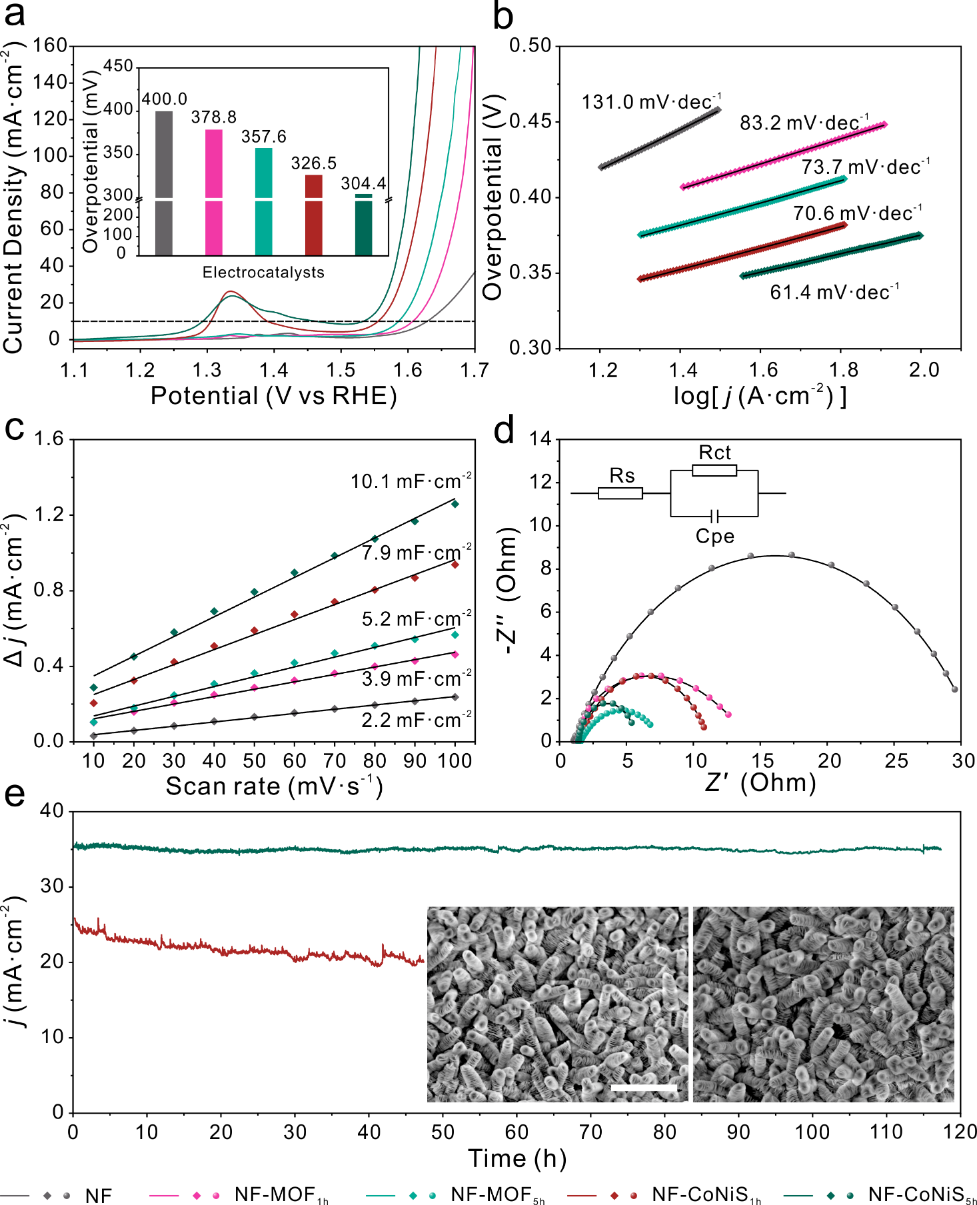
Figure 4: Electrocatalytic activity
This work was supported by the National Natural Science Foundation of China (21925405, 22104141, 22104142, 22004122), the National Key Research and Development Program of China (2018YFA0208800), the Chinese Academy of Sciences (XDA23030106, YJKYYQ20180044), and the China Postdoctoral Science Foundation (2020M680676, 2021T140680).